4 Compelling reasons why iPSC-derived hepatocytes are better than primary hepatocytes
In the pursuit of robust, scalable disease models, induced pluripotent stem cells (iPSCs) have enormous potential for drug discovery. While the long-standing use of immortalized cells and primary cells has yielded valuable results, the persistently high clinical trial failure rates in recent years have driven a search for alternatives. In this article, we outline how transitioning from primary cells to human iPSCs can unlock rapid, scalable, and human-relevant disease models to accelerate and de-risk drug discovery.
Transitioning from primary cells to iPSC-derived models
Across the drug discovery process, researchers look to utilize more human-relevant models to test the efficacy and action of their therapy. In early-stage research, the use of cultured and highly characterized cell types (such as Human Embryo Kidney, or HEK cells and overexpressed gene cell lines) is commonplace, due to ease of access, licensing, handling, and cost. Primary cells, cells taken directly from living tissue and established for growth in vitro, are a common next step. As primary cells are taken from human tissue, they represent physiologically relevant cell sources and can provide value for drug discovery. However, there are a few key drawbacks to using primary cells:
- Cell material can be costly and difficult to obtain.
- There can be difficulties in accessing primary cell material in the volume and donor consistency needed for high-throughput drug discovery.
- Primary cells can deteriorate and dedifferentiate in just several days once thawed and cultured.
With a growing need for robust and scalable cell models, human iPSCs are positioned as the “next step” toward human-relevant models with the utility to match the scale needed for drug discovery.
The use of human iPSC-derived cells for accessible, scalable disease models
Human iPSCs are stem cells with the capability of being differentiated into adult cells, such as hepatocytes, cardiomyocytes and neurons. The production of iPSCs starts from donated material from patients (most often blood samples) and provides a master ‘line’ from which a variety of cell types can be produced in bulk – making the cells both scalable while still being sourced from a single donor, thus removing the donor variation seen with primary cells.
While primary hepatocytes are usually metabolically competent, reflecting the same profile of receptors as human hepatocytes. The same cannot be said of all immortalized cell lines. Ulti-HEP, the iPSC-derived hepatocytes offered by DefiniGEN, are highly characterized and mature, demonstrating the functional membrane localization and activity of the Asialoglycoprotein receptor 1 (ASGR1). This level of maturity is necessary for researchers as it allows them to study how their compound would be received by healthy hepatocytes in vitro to generate reproducible data.
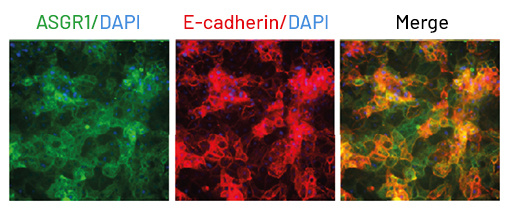
Fig 2: Ulti-HEP demonstrate functional membrane localization and activity of the Asialoglycoprotein receptor 1 (ASGR1).
Furthermore, iPSC-derived cells are amenable to genetic editing before differentiation, enabling the creation of isogenic models for studying specific genetic variants. This is particularly useful for elucidating disease mechanisms and validating drug targets. For example, iPSC-derived cardiomyocytes have been used to model inherited cardiac arrhythmias, providing insights into disease progression and therapeutic responses.
iPSC-derived cells are also capable of being cultured in vitro for much longer periods of time than primary cells. This advantage shouldn’t be overlooked, especially if the goal is to study toxicity of low clearance drug candidates or test novel compounds over long periods of time. This has been shown in iPSC derived cells such as hepatocytes, which do not dedifferentiate or deteriorate for weeks, unlike their primary hepatocyte counterparts, which can only be cultured for up to a week once thawed.
For these reasons, iPSC-derived models are now being thought of as a more cost-effective model to model diseases and develop drugs. Although the initial costs of generating iPSC-derived cells can be high, especially if undertaken by researchers who are inexperienced with iPSCs, their scalability and ability to reduce late-stage drug failures make them a cost-effective choice over time. By identifying potential safety or efficacy issues earlier, they help minimize the enormous expenses associated with failed clinical trials.
In summary, human iPSCs have four key advantages over primary cells: they are easily sourced, readily available, can be cultured for longer, and can be produced on far larger scales to support high-throughput drug discovery. With high-quality, physiologically relevant cells at the core of models, iPSCs are supporting biopharma on the drug discovery journey.
For more information about how Ulti-HEP can transform your research by driving new insights, visit our Cell Products page, or or you can contact one of our experts today.

