iPSC-derived Hepatic Stellate Cells
DefiniGEN have generated the first commercially available iPSC-derived HSCs
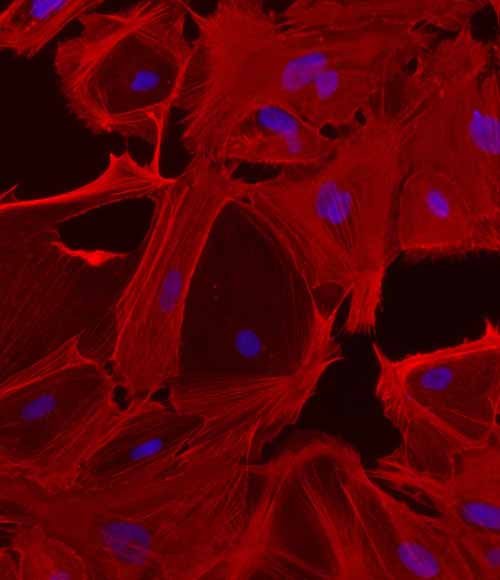
The liver is an organ of crucial importance, playing a central role in metabolism, detoxification, and immune function. While the primary cell type in the organ are parenchymal hepatocytes, other non-parenchymal cells are key to its functionality.
Hepatic stellate cells (HSC) are pericyte-like cells that reside within the liver sinusoids, in an area known as the space of Disse. Recognized in healthy livers for their role in storing vitamin A, HSCs exhibit remarkable plasticity, transitioning between quiescent and activated states in response to liver injury.
Chronic liver injury can result in fibrosis, which subsequently leads to cirrhosis and hepatocellular carcinoma. Because this dysregulated HSC activation underlies the pathogenesis of hepatic fibrosis, HSCs are a potential area to target to identify novel therapeutics for alleviating liver fibrosis and restoring hepatic homeostasis.

Limitations in Animal, Primary Cell and Immortalized Cell Models
Primary human hepatic stellate cells
Primary human hepatic stellate cells (pHSCs) are considered the gold standard in vitro model for fibrosis-related studies. However, these cells are difficult to isolate, demonstrate heterogeneity among liver donors, and the considerable differences between the diverse isolation processes increases batch-to-batch variation. In addition, pHSCs rapidly lose their quiescent features, they have a limited life span, and yield contamination with inflamed liver macrophages is often observed.
Hepatic stellate cell lines
To overcome some of the limitations of pHSCs, several immortalized rodent and human hepatic stellate cell lines (e.g., HSC-T6, LX-2) have been developed. These cell lines express smooth muscle actin filaments (α-SMA), esterify retinol, and respond to mediator of liver fibrogenesis (e.g., TGF-1), thus offering good HSC model alternatives. However, these cell lines show an activated HSC phenotype in culture, lack key HSC functional characteristics, and are prone to genotypic and phenotypic drifts following pro-longed culture and passage. In addition, rodent-derived HSC lines demonstrate species-specific differences, downstream compromising data translatability.
Key Features and Benefits of Our Hepatic Stellates
The development of induced pluripotent stem cells (iPSCs) has created a new opportunity in in vitro cell modelling, offering a promising alternative for the generation of fully functional liver cell types with unlimited expansion capacity, phenotypic stability and healthy karyotype.
DefiniGEN have generated the first commercially available iPSC-derived HSCs, which demonstrate the following advantages:
High purity
Our iPSC-derived hepatic stellates are not sourced from tissue and therefore aren’t mixed with other cell types
Single donor
Sourced from a single donor: Ensuring there is no batch-to-batch variation
Quiescent status
Which can be activated upon treatment with liver fibrogenesis mediators (TGF-1)
Cell markers
Expression of key hepatic stellate cell markers: e.g., Desmin, GFAP, PDGFRs, α-SMA and Collagen I
Collagen I secretion
Healthy karyotype
Technical data
Figure 1: Key HSC markers
DefiniGEN iPSC-derived Hepatic Stellate Cells (Ulti-HSC) express key HSC markers at gene and protein level

Figure 1A: mRNA levels of HSC markers (Desmin, PDGFRb, ALCAM, PCDH7, ACTA2, and COL1A1) in DefiniGEN Ulti-HSC following 2 weeks of iPSC differentiation and primary HSC (pHSC).

Figure 1B: Protein levels of the key HSC markers Desmin and GFAP in DefiniGEN Ulti-HSC.


Figure 2: Quiescent Ulti-HSC
Quiescent Ulti-HSC are successfully activated using pharmacological and mechanical stimuli

Figure 2A: Simplified timeline of the differentiation and activation of iPSC-derived Ulti-HSC using pharmacological and mechanical stimuli (TGFβ treatment or plastic attachment).

Figure 2B: mRNA levels of the HSC-activation marker COL1A1 in vehicle-treated, TGFβ-treated, or re-plated DefiniGEN Ulti-HSC for 5 days.

Figure 2C: Protein levels of Collagen I in vehicle-treated, TGFβ-treated, or re-plated DefiniGEN Ulti-HSC for 5 days. Data are presented as mean±SD of n=3 technical replicates. mRNA expression data were normalized to 18S rRNA. Cells were counterstained with DAPI.


Figure 3: Maintain cell signature
iPSC-derived hepatic stellate cells maintain their cell signature post-cryopreservation

Figure 3A: 10x objective. >85% cell viability. Standard hepatic stellate morphology. >85% double positive cells for GFAP and αSMA.
DefiniGEN cryopreserved iPSC-derived hepatic stellate cell morphology 24 hours post thaw.

Figure 3B: Protein levels of the key HSC markers GFAP, αSMA, and Collagen 1 in cryopreserved DefiniGEN Ulti-HSC.


