3 Key advantages to using DefiniGEN’s MASLD Model for Drug Discovery
Understanding Metabolic dysfunction-associated steatotic Liver disease (MASLD)
Metabolic dysfunction-Associated Steatotic Liver Disease (MASLD) represents a growing health concern, encompassing conditions from hepatic fat accumulation to more severe manifestations like steatohepatitis (MASH), fibrosis, cirrhosis, and even hepatocellular carcinoma. For researchers aiming to better understand this disease and discover effective treatments, DefiniGEN's MASLD model offers a cutting-edge in vitro platform. In this article, we will look at the current models employed to study MASLD and how our disease model can overcome some of the drawbacks associated with them.
MASLD spans a spectrum of liver conditions rooted in metabolic dysfunction. The disease begins with fat buildup within hepatocytes, progressing to inflammatory stages, as seen in MASH, and eventually leading to fibrosis and cirrhosis. Without early intervention, MASLD can result in liver failure, cancer, and eventually death. Traditional animal models and primary human liver cells have provided some insights into the disease, but they often fail to mimic the human metabolic and inflammatory processes central to MASLD. In turn, this leads to compounds being selected during the drug development process that fail to make it to the market.
Challenges with current MASLD models
Animal Models
Although murine models have contributed to MASLD research, they cannot fully replicate the complexity of human liver metabolism. High fat-enriched diets and genetic modifications in mice induce MASLD-like symptoms, but these approaches often fail to translate into meaningful human clinical outcomes. This leads to prospective drug candidates failing to pass clinical trials and results in pharmaceutical companies losing time and money.
Primary Cell Models
Human liver cell cultures, including primary hepatocytes, offer more relevance to human physiology, particularly in the study of inflammation and lipid metabolism. However, challenges such as limited cell lifespan, donor variability, and loss of function over time reduce their effectiveness, especially in high throughput or long-term studies. Without the ability to carry out high throughput screening investigations, researchers are forced to source primary cells from several donors which leads to a high variability rate and difficulties in reproducibility.
Immortalized liver cell lines
Immortalized liver cell lines, such as HepG2 cells, are commonly used in MASLD research due to their ease of use and indefinite proliferation. However, these cell lines originate from cancerous tissue, which compromises their ability to accurately represent normal liver function. HepG2 cells, for example, exhibit low fatty acid oxidation, limiting their relevance for studying key metabolic processes in MASLD. In addition to these limitations, immortalized cells are characterized by the presence of the PNPLA3 I148M mutation - a MASLD risk-associated variant - as well as low drug-metabolizing activity, further compromising their use as a physiologically relevant in vitro liver system.
DefiniGEN’s MASLD model: A new benchmark
Through our UltiDIFF platform, we have developed an in vitro MASLD model that addresses and overcomes the limitations of models mentioned previously. Ulti-HEP are human iPSC-derived hepatocytes that combine the expansion capacity of immortalized cell lines and the healthy phenotype of primary human hepatocytes. Crucially, these cells accurately recapitulate the key aspects of MASLD when exposed to a high fat and sugar diet, including lipid accumulation, insulin resistance, lipotoxicity, inflammation, and compromised drug-metabolizing activity. These key features makes our model a highly predictive and scalable platform for drug discovery and mechanistic studies.
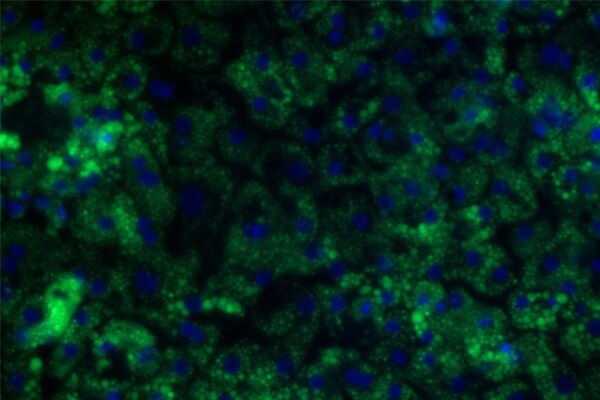
When our Ulti-HEP cells are treated with high glucose or high fat diets, they demonstrate increased lipid accumulation, insulin resistance, lipotoxicity and inflammation. This makes them an accurate and ideal human derived model to screen compounds in high throughput investigations.
Key Advantages of the Ulti-HEP MASLD Model
- Scalability and Reproducibility
Sourced from a single donor, the Ulti-HEP model offers consistency and eliminates batch-to-batch variability. This scalability is essential for high-throughput drug screening and testing, ensuring reliable data across experiments.
- Phenotypic Relevance
When treated with high-sugar or high-fat diets, Ulti-HEP mirror the key features of MASLD, including increased lipid accumulation, insulin resistance, and elevated pro-inflammatory cytokine markers. This makes it an accurate platform for evaluating therapeutic candidates. For more information, click here.
- Better predictive outcomes
Unlike animal models, Ulti-HEP are derived from human iPSCs, making the findings more translatable to clinical settings. This reduces the risk of unsuitable compounds advancing in drug development pipelines.
Insights from the MASLD model
Lipid Accumulation
Lipid buildup is one of the early hallmarks of MASLD. When Ulti-HEP are exposed to high glucose and free fatty acids, they exhibit significant lipid accumulation, as demonstrated through BODIPY staining. This reliable replication of steatosis makes Ulti-HEP an ideal model for exploring therapeutic interventions aimed at reducing hepatic lipid content.
Insulin Sensitivity
Insulin resistance is a defining feature of MASLD and MASH. Ulti-HEP, when treated with high glucose, show reduced phosphorylation of AKT — a key protein in the insulin signaling pathway. This demonstrates their impaired response to insulin, providing an accurate in vitro model for studying insulin resistance and testing insulin-sensitizing drugs.
Lipotoxicity and Inflammation
Lipotoxicity, or the harmful effects of lipid accumulation on cell function, is closely linked to inflammation in MASLD. High fat-treated Ulti-HEP display reduced viability and elevated expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNFα. This makes our model well-suited for studying inflammatory pathways in MASLD and screening anti-inflammatory drugs.
Gene-edited MASLD models: The role of PNPLA3 I148M mutation
Recent genome-wide association studies (GWAS) have identified specific genetic variants associated with MASLD progression. One such variant is the I148M mutation in the PNPLA3 gene, which has been shown to exacerbate liver fat accumulation and inflammation. We have utilized CRISPR/Cas9 technology to create gene-edited Ulti-HEP disease models that carry the PNPLA3 I148M mutation, providing researchers with a unique tool to study the genetic aspects of MASLD and develop targeted therapies.
DefiniGEN’s MASLD model represents a breakthrough in liver disease research and drug discovery. Offering scalability, reproducibility, and phenotypic accuracy, this in vitro platform addresses the limitations of traditional models and provides a robust solution for understanding MASLD and developing new treatments. Whether you are investigating lipid metabolism, insulin resistance, or the genetic underpinnings of MASLD, Ulti-HEP offers an optimized solution for high-throughput screening and mechanistic studies.
To learn more about our MASLD model, speak to one of our experts today

