Discussing DefiniGEN's iPSC-derived MASLD model with Head of R&D, Nikolaos Nikolaou
In this interview, Marketing Manager Haris Choudhery, PhD, sat down with Head of R&D Nikolaos Nikolaou, DPhil, to discuss Our in vitro MASLD model represents an optimized cell platform for drug discovery applications and a principal tool for elucidating the underlying mechanisms of the disease.
Hi Nik, tell us about your background and how you came to work for DefiniGEN
Hi Haris, thank you for the invite. My name is Nik Nikolaou, and I am the Head of Research & Development at DefiniGEN Ltd. I have been with DefiniGEN for the last 2 and a half years. I am a liver biologist by training, and I completed my DPhil at the University of Oxford focusing on the role of steroid metabolome in the pathogenesis of Metabolic-dysfunction Associated Steatotic Liver Disease (MASLD). Following this, I was a Postdoctoral Research Associate at the University of Oxford working on the characterization of bile acid signaling in the pathogenesis of MASLD-related liver cancer, before moving to the University of Cambridge to investigate the role of immune-mediated steroidogenesis in the same disease. Since 2022, I have been with DefiniGEN Ltd. and driving the scientific strategy of the company.
What is Metabolic-dysfunction Associated Steatotic Liver Disease (MASLD)? And how does it affect the body?
MASLD is the hepatic manifestation of the Metabolic Syndrome. It is a spectrum of disease ranging from lipid storage within liver cells, also known as steatosis, to steatohepatitis with associated inflammation (MASH), and can eventually progress to the development of fibrosis, cirrhosis, and even liver cancer (Fig. 1). It is a silent disease, as many individuals with MASLD do not experience noticeable symptoms, especially in the early stages, however, it affects more than 25% of the population worldwide. Despite the severity, there is currently only one licensed treatment for MASH.
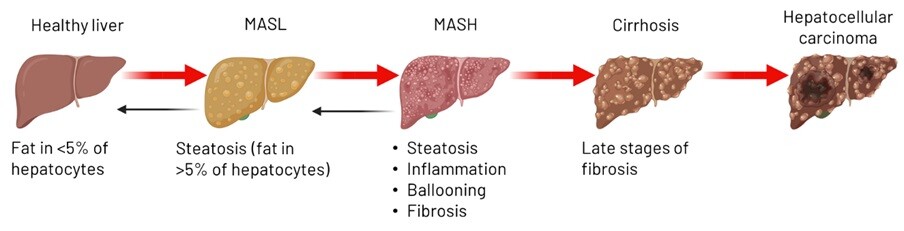
Figure 1: Schematic demonstrating the spectrum of Metabolic-dysfunction Associated Steatotic Liver Disease (MASLD). Made with Biorender.
What tools or models do researchers use to study MASLD?
MASLD is characterized by high complexity and heterogeneity. A wide range of both in vivo and in vitro models have been developed over the last 10-15 years, trying to understand this disease pathophysiology. A variety of dietary rodent models, including high-fat, high-glucose, or methionine/choline deficient diets are commonly used to recapitulate disease phenotype in body organisms accompanied by genetic models of MASLD, where mutations in key metabolic-related genes are introduced to drive aspects of the disease, including steatosis and insulin resistance. In parallel, to understand the molecular cues and identify novel cellular targets for therapeutic development, different cellular models are currently used, including primary human cells, human hepatic cell lines, or human precision-cut liver slices.
Are there any drawbacks to using these models?
Despite the advantages the above tools can offer in MASLD research, there are several limitations that compromise model predictivity and data reproducibility. In vivo models of MASLD do not accurately recapitulate human physiology due to species-specific differences – as an example, rodents show different metabolic responses to humans, especially with regards to lipid and glucose metabolism, thus limiting the applicability of the findings. At the same time, current in vitro models, in particular primary human cells, show uncontrolled donor variability and rapid loss of metabolic function once in culture, while liver cell lines are often of cancerous origin and fail to show crucial metabolic capabilities, such as adequate fatty acid oxidation or drug metabolism.
How does DefiniGEN's iPSC-derived hepatocytes models, Ulti-HEP, overcome these drawbacks?
By leveraging the benefits induced pluripotent stem cells (iPSCs) offer with regards to expansion capacity and healthy phenotype, we have generated highly functional hepatocytes that overcome the limitations of the current cell models. Using these iPSC-derived hepatocytes, we have developed a reproducible in vitro MASLD model that replicates the disease phenotype in-a-dish (e.g., lipid storage, insulin resistance, inflammation) (Fig. 2), and this can be used for not only the large-scale screening of novel therapeutics against the disease, but also further elucidating disease pathophysiology.
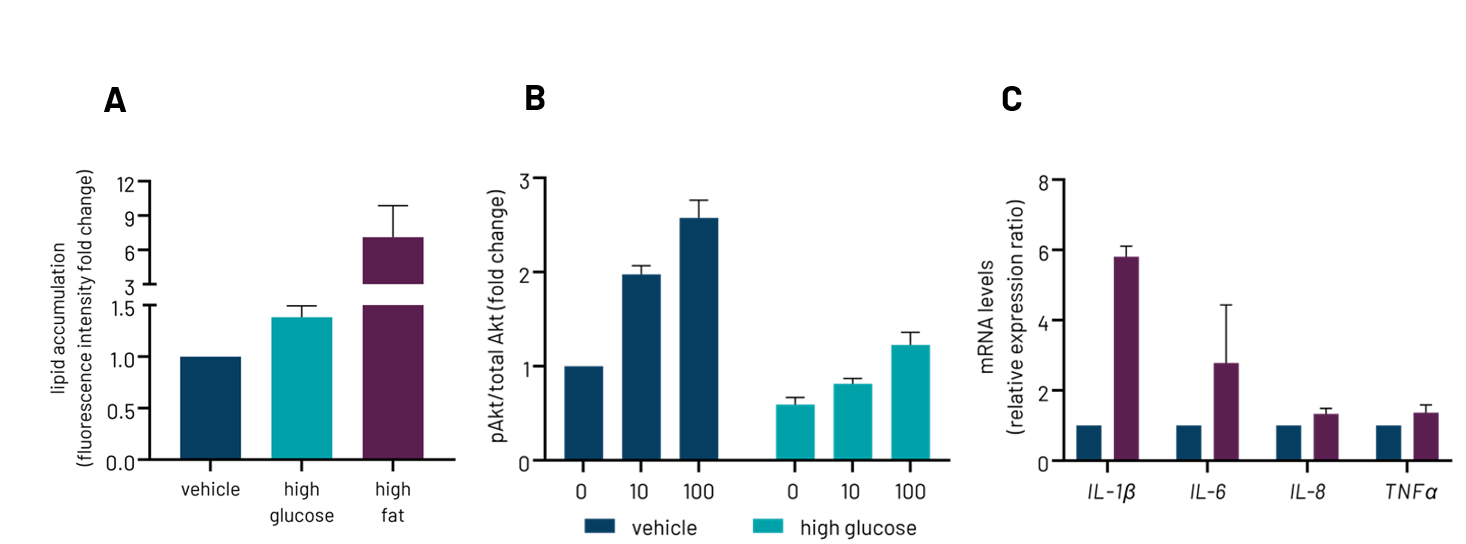
Figure 2: DefiniGEN wild type Ulti-HEP demonstrate features of Metabolic-dysfunction Steatotic Liver Disease when put under high glucose or high-fat diet, including steatosis (A), insulin resistance (B), and inflammation (C).
Additionally, we have recently developed hepatic stellate cells derived from the same iPSC donor, aiming to understand additional aspects of the disease spectrum, in this case fibrosis, and how different liver cell types of the same genetic background interact to drive disease progression. Last, but not least, iPSCs allow us to introduce risk-associated MASLD mutations via CRISPR/Cas9 gene editing (e.g., PNPLA3, TM6SF2, HSD17B13) offering the opportunity to also study the role of genetic determinants in MASLD, as well as other genetic liver diseases.
Ready to enhance your drug screening capabilities with iPSC-derived models and high-throughput scalability? You can learn more about our MASLD model here, or you can contact one of our experts today.

