Toxicology Screening Services
Ulti-HEP are an optimal platform for predicting hepatic safety and measuring drug toxicity
Limitations in toxicology
Primary human hepatocytes, animal models, and hepatocellular carcinoma cell lines have significant limitations in toxicology testing of potential drug compounds
Primary cells
Primary cells exhibit donor variability and limited lifespan, leading to inconsistent results1
Animal models
Animal models, while useful, do not always accurately predict human responses due to species-specific differences in metabolism and physiology2
Carcinoma cell lines
Carcinoma cell lines have altered genetic and metabolic pathways that do not reflect normal human cell behavior, thus failing to provide reliable toxicity data3.
Due to these imperfect models, most therapeutics fail to reach clinical trials due to the lack of translatability between these inadequate pre-clinical models and the clinic.
These limitations underscore the need for better physiologically relevant models for accurate and predictive toxicology screening. For this reason, DefiniGEN have created induced pluripotent stem cell (iPSC)-derived hepatocytes, Ulti-HEP, to give researchers a new model that overcomes these drawbacks and de-risks drug discovery by providing a new and highly predictive in vitro toxicology screening platform.

Advantages of Ulti-HEP for Toxicology Screening
Our proprietary iPSC-derived Ulti-HEP cells are phenotypically relevant and offer better predictive insights in hepatotoxicity testing, helping you to determine the hepatotoxic potential of both novel drug compounds and gene therapy delivery systems early in your drug discovery pipeline.
Predictive accuracy
Unlike animal models, our Ulti-HEP closely mimic human liver function, providing predictive and translatable data.
No batch-to-batch variation
Our iPSC-derived hepatocytes are sourced from a single donor, removing batch-to-batch variation and enhancing data reproducibility.
Genetically superior to carcinoma cell lines
Our Ulti-HEP do not carry abnormal genetic karyotypes unlike carcinoma cell lines such as HepG2.
Scalability
Suitable for high-throughput screening, enabling large-scale testing with consistent, reliable results. We are able to scale to meet your needs.
Regulatory Compliance
Generate robust data that meets regulatory requirements, facilitating smoother approval processes.
Customized Solutions
Our team works closely with you to develop bespoke cell models and screening strategies that address your unique challenges.
Key Features of Ulti-Hep
Ulti-HEP represents a game changing model for toxicology screening. Utilizing our proprietary iPSC technology, we produce phenotypically-relevant hepatocytes that recapitulate key liver functions, enabling precise assessments of drug-induced hepatotoxicity.
Human-relevant models
Derived from induced pluripotent stem cells (iPSCs), our Ulti-HEP cells exhibit all human-specific metabolic pathways, giving you relevant and reproducible data, leveraging the consistency of iPSC culture.
Functional assays
Evaluate key liver functions such as cytochrome P450 enzyme expression, activity, inhibition, and induction.
Efflux transporter expression
Ulti-HEP express functional levels of the efflux transporters required for large-scale toxicity screening. Our cells demonstrate the cellular localization of these transporters in the hepatocyte membrane, confirming their functional role in bile acid and drug transport.
Scalability
Suitable for high-throughput screening, enabling large-scale testing with consistent, reliable results.
Ulti-HEP Functionality data

Figure 1a: Phase I CYP450 genes
mRNA expression levels of Phase I CYP450 genes in liver carcinoma HepG2 cells, primary human hepatocytes and DefiniGEN Ulti-HEP.

Figure 1b: Basal CYP3A4 activity
Basal CYP3A4 activity in liver carcinoma HepG2 cells, DefiniGEN Ulti-HEP, and primary human hepatocytes (PHH). mRNA data were normalised to the housekeeping gene 18SrRNA and are presented as mean±SEM of n=3-4 independent experiments. CYP3A4 activity data were normalised to ATP levels and are presented as mean±SEM of n=3-5 independent experiments. For PHH data, cells from 3 independent donors were used.
Cytochrome P450 expression and activity
The cytochrome P450 (CYP) enzymes are the major players in drug metabolism, being responsible for the metabolism of >90% of the drugs that are currently used in clinic. Primary human hepatocytes are considered the gold standard in vitro model in ADME and toxicology screening, however, they come with limitations, including short-life span, rapid loss of function, and limited supply.
Due to these limitations, hepatocellular carcinoma cell lines, including HepG2 and HepaRG, are instead used. However, the malignant origin of these models, in addition to the lack of demonstrable drug metabolizing enzyme expression, hinders data interpretation.
DefiniGEN’s improved differentiation iPSC protocols now lead to the generation of Ulti-HEP with functional CYP450 enzyme expression and activity. In Figure 1, we demonstrate that Ulti-HEP express significantly higher mRNA levels of various CYP enzymes compared to HepG2 carcinoma cells, including CYP3A4, CYP2B6, CYP2C9, CYP2C19, and CYP2A6. Importantly, we show the activity levels of CYP3A4 in DefiniGEN Ulti-HEP, revealing comparable functionality to that seen in primary human hepatocytes and significantly higher to that of HepG2 cells.
Cytochrome P450 induction and inhibition
Induction and inhibition of CYP450 enzymes are central mechanisms in xenobiotic metabolism, resulting in pharmacokinetic drug-drug interactions (DDI). Both mechanisms are of particular clinical importance for therapeutic and toxicological reasons, as inhibition of drug metabolism can lead to undesirable elevations in plasma drug concentrations, whilst CYP induction following prolonged drug treatment has been associated with adverse DDI. Thus, access to in vitro models that can accurately predict both mechanisms is crucial in drug therapy.
Here we show that, unlike HepG2 cells, CYP450 activity can be both induced and inhibited in DefiniGEN Ulti-HEP at comparable levels to those seen in primary human hepatocytes following treatment with known inducers and inhibitors.
As proof-of-concept, we demonstrate CYP3A4 induction in Ulti-HEP following treatment with the CYP3A4 inducers Rifampicin and 1α,25-hydroxy-vitamin D3 as well as CYP3A4 activity in HepG2, Ulti-HEP, and primary human hepatocytes following inhibition with the CYP3A4 inhibitor ketoconazole and induction with increasing concentrations of the CYP3A4 inducer 1a,25-hydroxy-vitamin D3 (Figure 2a).

Figure 2a: CYP3A4 induction and inhibition in liver carcinoma HepG2 cells, DefiniGEN Ulti-HEP and primary human hepatocytes (PHH), following 72h of treatment with vehicle, 1μM ketoconazole (CYP3A4 inhibitor), 10-100nM vitamin D3 (CYP3A4 inducer), or a combination of vitamin D3 and ketoconazole. CYP3A4 activity data were normalised to ATP levels and are presented as mean±SEM fold change of n=3-5 independent experiments. For PHH data, cells from 3 independent donors were used.

Figure 2b: CYP3A4 mRNA induction in DefiniGEN Ulti-HEP following 72h of treatment with vehicle, 50μM Rifampicin, or 100nM vitamin D3 (CYP3A4 inducers).

Figure 2c: Albumin secretion in DefiniGEN Ulti-HEP following 48h of treatment with increasing concentrations (0-250 μM) of 7 compounds of known DILI liability.

Figure 3a: Efflux transporter
mRNA expression levels of efflux transporter genes ABCB11, ABCG2, and MRP2 in liver carcinoma HepG2 cells and DefiniGEN Ulti-HEP. mRNA data were normalised to the housekeeping gene 18SrRNA and are presented as mean±SEM of n=3-4 independent experiments.

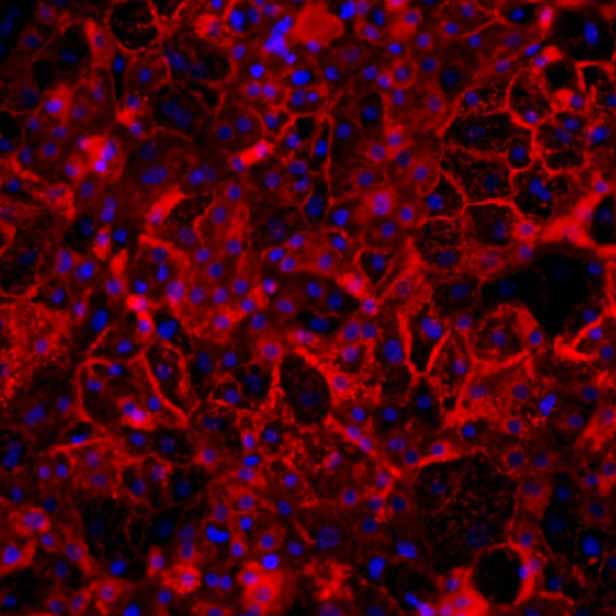
Figure 3b:
Protein expression of efflux transporter proteins ABCB11 and MRP2 (green), co-stained with the apical marker ZO1 (red) in sandwich-cultured Ulti-HEP by immunocytochemistry (ICC). Nuclei counterstaining with DAPI (blue).
Efflux transporter expression and cellular localization
In addition to the central role of CYP450 enzymes in drug metabolism, inhibition of liver-specific efflux transporters, including the bile acid export pump (BSEP/ABCB11) and the multidrug resistance (MDR) proteins (e.g., ABCG2, ABCC2/MRP2) has emerged as a DILI risk factor. Supporting this, in vitro screening for BSEP inhibition in drug discovery is now recommended by the European Medicines Agency on the Investigation of Drug Interactions (2012).
Despite the need of physiological hepatic cell systems for the accurate in vitro screening of efflux transporter inhibition, the predominant in vitro models that are currently used include non-hepatic cell lines (e.g., HEK293) that artificially overexpress a single transporter, only, since transporter expression in hepatocellular carcinoma cells is either absent or lack proper cellular localization.
Here, we show that DefiniGEN’s improved Ulti-HEP express functional levels of the efflux transporters required (e.g., ABCB11, ABCG2, MRP2) for large-scale toxicity screening. Crucially, we demonstrate the cellular localization of these transporters in the hepatocyte membrane, confirming their functional role in bile acid and drug transport (Figure 3).
Drug-Induced Liver Injury (DILI) predictivity
Drug-induced Liver Injury (DILI) is a leading cause for drug failure in clinic, accounting for more than 50% of liver failure cases. DILI is usually the result of poor suitability of the available pre-clinical models to predict toxicity of positive compounds, either due to species-specific differences (as seen in animal models and animal-derived cell lines), donor-to-donor variability and short-life span (as seen in primary human hepatocytes), or low metabolic capacity (as seen in hepatocellular carcinoma cell lines).
Recently, efforts to address the short-life span of primary human hepatocytes have resulted to the generation of 3D in vitro liver models with promising results. However, these models still fail to address the donor-to-donor variability issue, limiting their suitability for high-throughput screening during the early phases of the discovery process.
Here, we assessed DefiniGEN’s Ulti-HEP ability to accurately predict toxicity risk for a broad set of compounds with known DILI liability. The results revealed that Ulti-HEP can accurately predict DILI across a dose-response using cell viability and albumin secretion (ATP content) as endpoint assays, consistent with the functional CYP450 enzyme expression and activity demonstrated below (Figure 4). These data alongside the expansion capacity and lack of donor-to-donor variability iPSC-derived cell models offer highlight both the superiority of Ulti-HEP over the current hepatocyte models and their suitability in high-throughput hepatotoxicity screening.
In addition to the endpoint assays showed below, our cells can be used for the measurement of additional endpoint clinical biomarkers that are routinely tested during DILI screening, including urea and ALT upon request.

Figure 4: Cell viability (ATP content) in DefiniGEN Ulti-HEP following 48h of treatment with increasing concentrations (0-250 μM) of compounds with known DILI liability (classification based on Proctor et al. 2017). Cell viability data were normalised to positive (250 μM chlorpromazine) and negative controls (0.2% DMSO) and are presented as mean±SD of n=3 technical replicates.
References
- Ryu, B., Hong, S. M., Bang, D., & Kim, B. Y. (2021). Advantages and limitations of primary cells in human health. Biomedicines, 9(11), 1508.
- Hartung, T. (2019). Perspectives on In Vitro to In Vivo Extrapolations. Frontiers in Pharmacology, 10, 198.
- Barretina, J., Caponigro, G., Stransky, N., Venkatesan, K., Margolin, A. A., Kim, S., ... & Garraway, L. A. (2012). The Cancer Cell Line Encyclopedia enables predictive modeling of anticancer drug sensitivity. Nature, 483(7391), 603-607.


